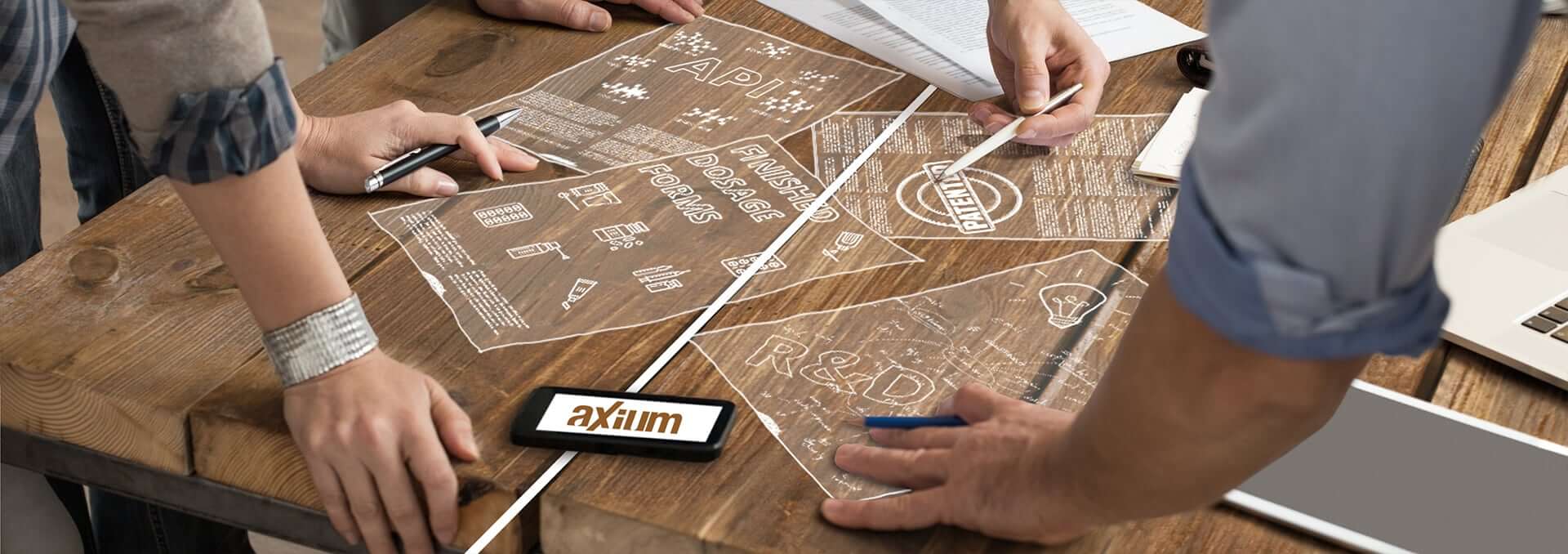
Development, licensing and supply of pharmaceutical products worldwide
Galenicum Axium is the new name for our well-established business-to-business operations within the Galenicum ecosystem, taking us one step closer to becoming a global specialty pharmaceutical company.
Under this new brand, our team will continue to strengthen our position in the Active Pharmaceutical Ingredients (API) field, as well as in Research and Development, Licensing and the Supply of high-quality affordable Finished Dosage Forms (FDFs) to other pharmaceutical companies around the globe.
We currently have commercial relationships with over 150 companies in over 50 countries around the world. And we are ready and keen to develop more!

Relentless passion for R&D...
At Galenicum Axium, we devote a significant amount of resources and energy to research and development (R&D) activities, with the aim of continually expanding and improving our product portfolio to the benefit of our clients and their patients.
Our Intellectual Property, R&D, Clinical and Regulatory teams do not only have significant technical expertise and know-how but also the superb interpersonal and creative skills needed to address any project.
The highest quality…
We apply strict processes and maximize the use of our systems across the organization to meet the highest quality standards for our products, at an affordable price.
Our Quality system is built to meet global quality standards and requirements, including the European Union, United States, Canada, Mexico and Brasil, amongst others.


A global portfolio...
We are pleased to be able to offer you a broad portfolio of Finished Dosage Forms, covering all therapeutic areas and various type of technologies.
We are more and more focused on innovation, which could bring an added value to doctors and patients, and complex products. Our aim is Galenicum Axium to become a referent in the development of pharmaceutical products worldwide.
100% customer-oriented...
At Galenicum Axium, we have a solid client orientation. Service and flexibility is a hallmark of the culture throughout Galenicum, encouraging these principles to apply in every interaction with our customers.
We also offer flexible partnership models. Whatever the form of partnership and cooperation you have in mind and whatever the stage of your project, we are always open to talk about how we can work together.