Midostaurin NCE-1
December, 2021
Galenicum partner files Midostaurin in the US
In 2021, Galenicum accomplished an important milestone with the submission of Midostaurin soft gel capsules 25mg to the US FDA on the NCE-1 date (April 2021).
- What is Midostaurin?
Midostaurin is an oral prescription medicine used to treat adults:
- Newly diagnosed with a certain type of acute myeloid leukemia (AML), in combination with certain chemotherapy medicines.
- With aggressive systemic mastocytosis (ASM), systemic mastocytosis with associated hematological neoplasm (SM-AHN), or mast cell leukemia (MCL).
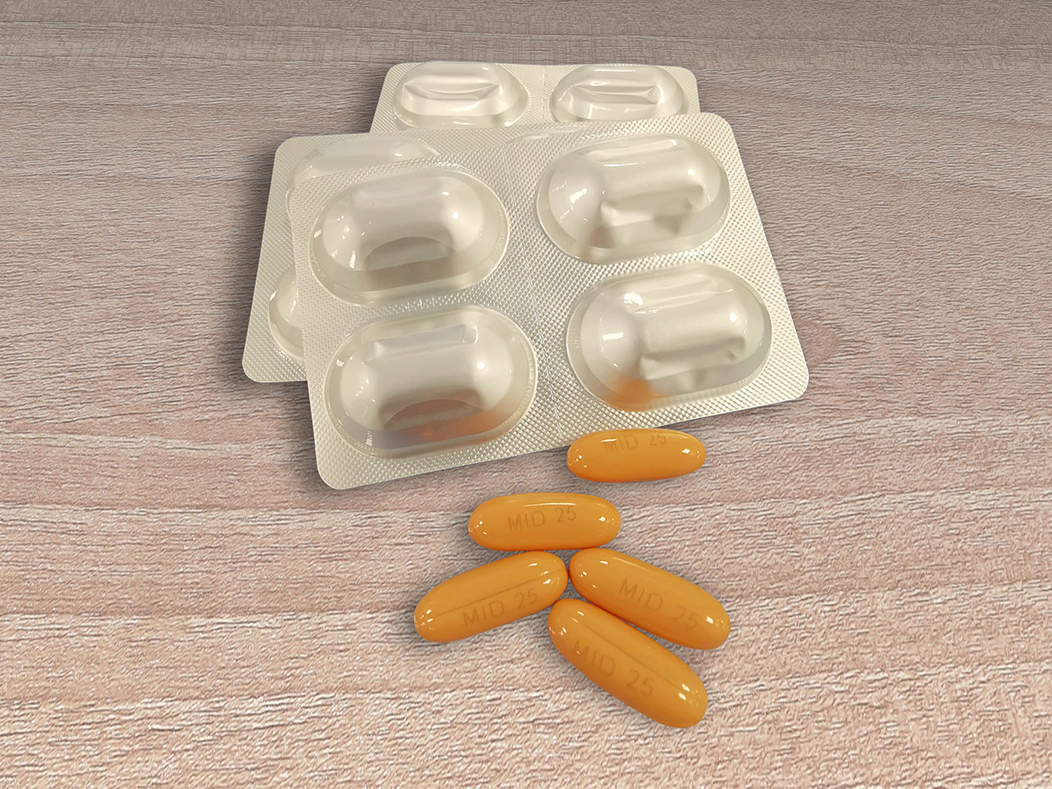
It is supplied as a soft gel capsule containing 25mg of Midostaurin.
- What is the NCE-1?
Indicates the first opportunity for generic drug companies to file Abbreviated New Drug Applications (ANDAs) for generic entry into the US market. Generic launch depends on many factors, including FDA approval and patents. This information is provided as a rough estimate of generic application, and does not indicate when generics will launch.
- Why is this milestone so important?
It is Galenicum's first oral dosage form submitted to the US FDA, with two successful bioequivalence studies required: Fast and Fed. The prior submissions done to the US FDA were all injectable products, fitting our strategy of offering niche and value-added products for this market.
Submitted on time. Deadline for submission was April, 28th, 2021, the NCE-1 date for the molecule. Having submitted the Dossier on this specific date may allow Galenicum and its partner to opt for a potential 6 months exclusivity period on the US market as a first generic, in the event registration process is completed successfully.
- What now?
Galenicum has already obtained the acceptance letter for evaluation of the FDA and is working with our partner to secure the approval at the earliest.
In parallel, Galenicum is also working to adapt the dossier for other geographies, such as the EU, and become a global player with this product.
Thanks to all our team and partners for this accomplishment and to make it possible. We are ready and keen to develop more!